History of TKTL1
Key Evolutionary Gene Opens New Frontiers in Research and Diagnostics
TKTL1—short for transketolase-like-1—is a gene discovered in 1995 by Dr Johannes Coy. For many years, it was largely overlooked.
Today, however, TKTL1 is gaining recognition as a pivotal player in evolutionary biology, cellular processes, and medical research. This gene is now understood to regulate the cell cycle, contribute to the cognitive advancements that allowed Homo sapiens to surpass Neanderthals, and drive the unchecked cell proliferation seen in cancer.
An Evolutionary Key Gene with Unimagined Potential
The 2022 Nobel Prize in Medicine, awarded to Swedish physician and biologist Svante Pääbo, brought further attention to TKTL1. By sequencing the Neanderthal genome, Pääbo identified a critical mutation in the TKTL1 gene that set modern humans apart. This mutation was instrumental in brain development, playing a significant role in the evolution of cognitive abilities unique to Homo sapiens.
As research progresses, TKTL1 has become a focal point for understanding not only human evolution but also its implications in diagnostics and therapeutic advancements. Its newfound significance ensures that TKTL1 will remain at the forefront of scientific exploration for years to come.
Two Sides of the Same Coin
TKTL1 serves a dual purpose: it protects both healthy and degenerated cells. This gene plays a critical role in safeguarding delicate cellular processes. For instance, it prevents retinal damage caused by excessive radicals, protects sperm cells from DNA mutations in the testicles, and ensures error-free cell division.
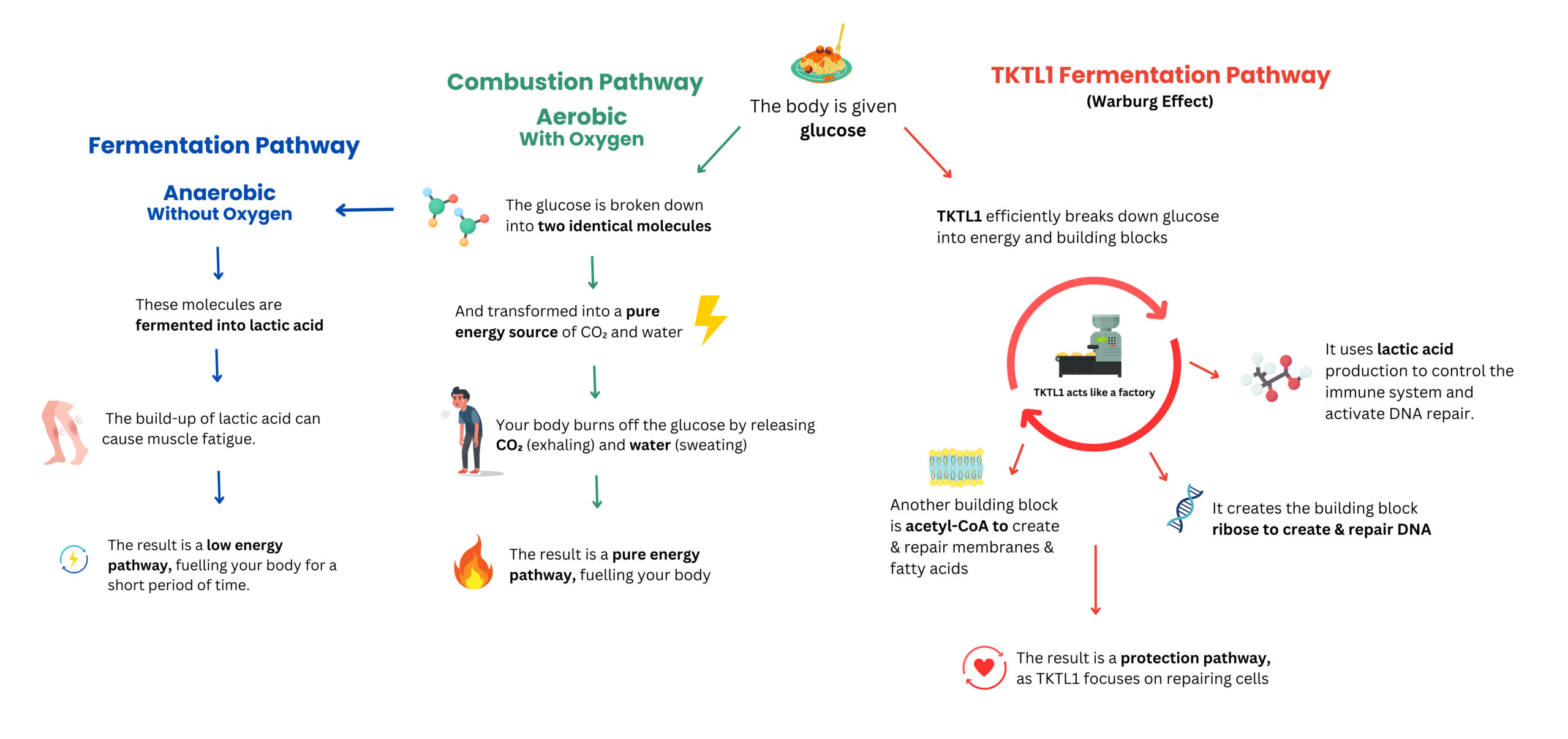
The protective mechanism lies in its ability to switch from aerobic mitochondrial metabolism—which generates energy but produces harmful free radicals—to anaerobic fermentation, a safer alternative that avoids radical formation. While fermentation produces less energy, this trade-off benefits cells during sensitive processes like division.
However, cancer cells exploit this same mechanism to their advantage, using anaerobic fermentation metabolism to support their growth and survival. This leads to several malignancy-related benefits:
Accelerated Growth:
TKTL1 facilitates the production of essential building blocks like nucleotides.
Immune Evasion:
Lactic acid, a byproduct of fermentation, creates a barrier that impairs immune cells.
Tissue Invasion (Metastasis):
Lactic acid damages surrounding tissue, enabling the tumour to spread.
Sustained Blood Supply:
TKTL1 stabilises HIF-1α, a molecule that promotes the formation of new blood vessels, ensuring a steady supply of nutrients to the tumour.
Inhibition of Programmed Cell Death:
TKTL1 reduces cytochrome c, a key molecule in apoptosis, allowing cancer cells to survive longer.
A Simple Introduction to the TKTL1 Fermentation Pathway
New Findings on the Warburg Effect
Dr Johannes Coy’s research has led to a reinterpretation of the “Warburg effect,” first described by Otto Warburg in 1923/1924. Coy discovered that the fermentation of sugar into lactic acid—anaerobic cell metabolism—is not exclusive to cancer cells, even when oxygen is present. This metabolic pathway also plays a protective role in healthy cells. For instance, it safeguards retinal cells exposed to intense sunlight and protects sperm cells from DNA mutations. Additionally, during the sensitive process of cell division, all cells switch to fermentation metabolism, a mechanism enabled by the activity of the TKTL1 gene.
Recognising TKTL1‘s diagnostic potential, Coy began developing a blood test based on this gene in 2007. His work culminated in the creation of the PanTum Detect® blood test, commercially available worldwide for early cancer detection.
